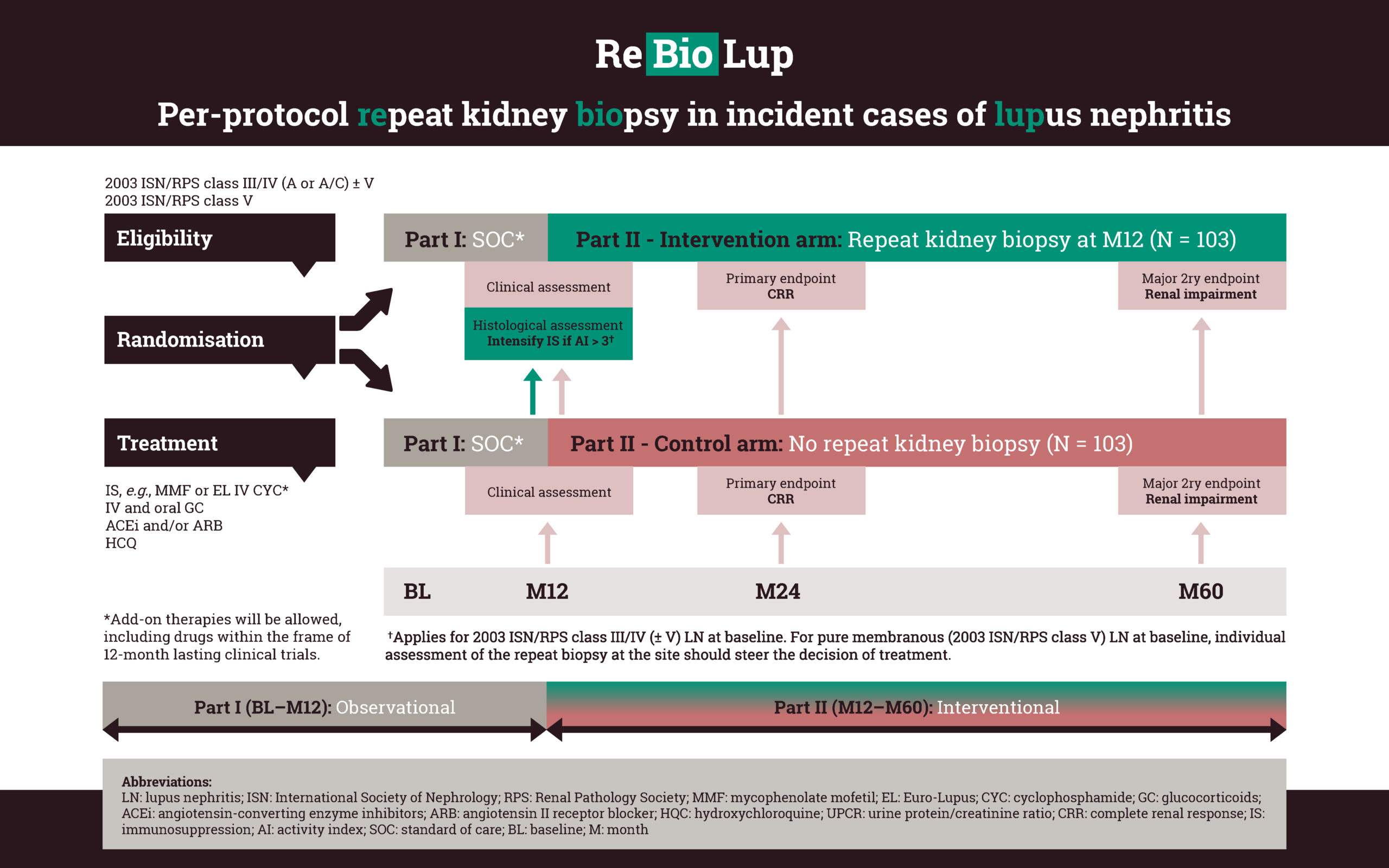
A prospective repeat kidney biopsy endeavour
This study is conducted within the frame of the Lupus Nephritis Trials Network, and is endorsed by the European Lupus Society (SLEuro) and the European Renal Association (ERA).
Objectives

to determine
the percentage of LN patients in histopathological remission after 12 months of standard of care immunosuppression.

to correlate
histological and immunological response to therapy (immune deposits) with clinical response.

to evaluate
whether therapeutic decisions steered by the results of a per-protocol repeat kidney biopsy improve renal outcomes compared with a matched control group of patients who did not undergo repeat kidney biopsy.

to generate data
on how to evaluate response to therapy in pure membranous LN (ISN/RPS class V), as well as the value of the information retrieved from repeat kidney biopsies in portending long-term renal prognosis in this LN subset.
Steering Committee
Ioannis Parodis (Stockholm, Sweden)
Farah Tamirou (Brussels, Belgium)
Julia Weinmann-Menke (Mainz, Germany)
Hans-Joachim Anders (Munich, Germany)
Brad H. Rovin (Columbus, United States)
Frédéric A. Houssiau (Brussels, Belgium)