Study Design & Flowchart
Study Design
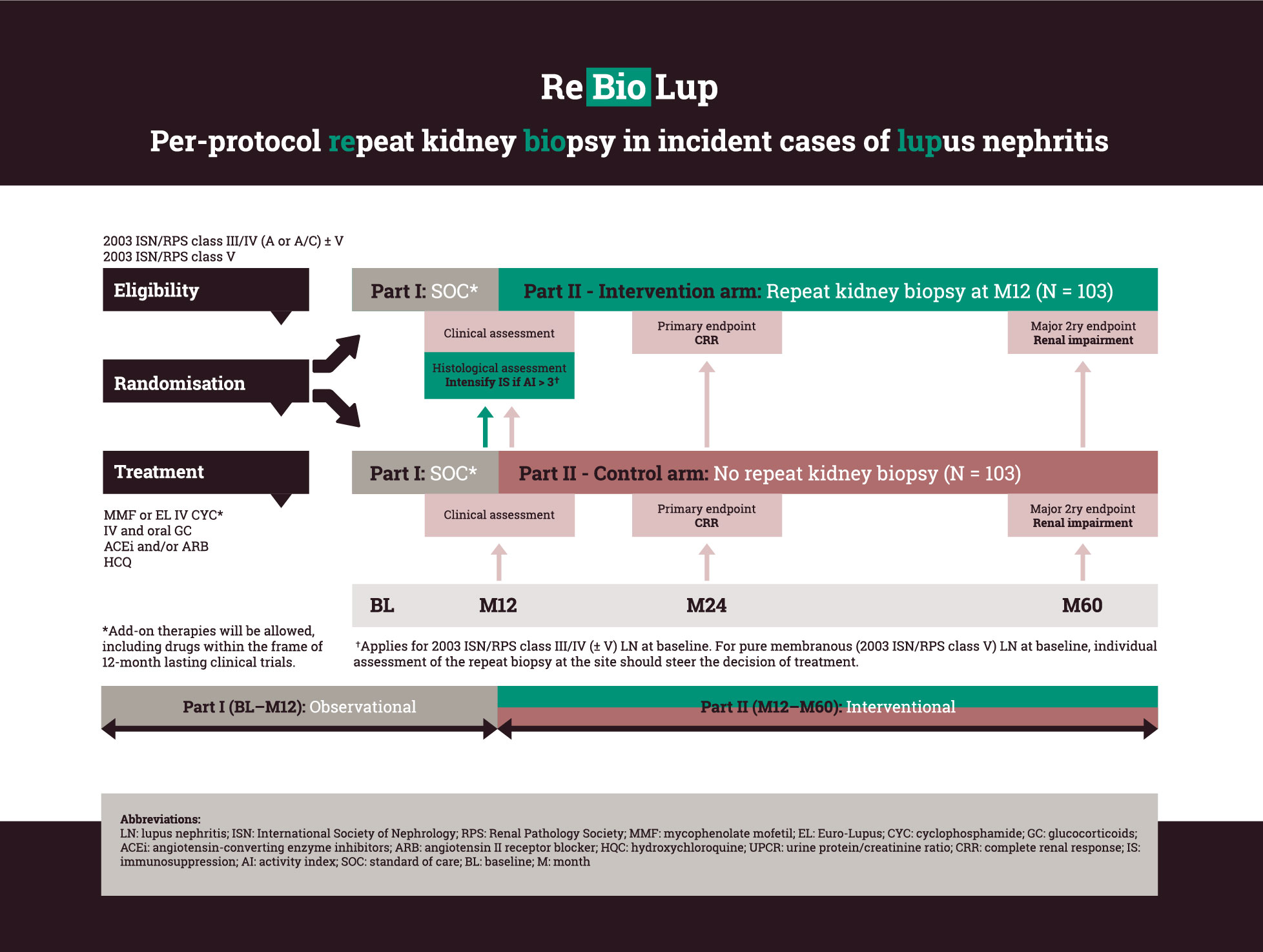
Patients with an incident biopsy-proven proliferative or membranous LN, or combinations thereof, selected to be initiated at standard of care immunosuppressive therapy with either MMF or EURO-Lupus IV CYC [Houssiau FA et al. Arthritis Rheum 2002] (combined with GCs and ACE inhibitors/ARBs) will be enrolled in this prospective study. Add-on therapies on the top of the aforementioned regimens will be allowed. At baseline, patients will be randomised 1:1 to either undergo or not undergo a per-protocol repeat kidney biopsy at month 12 from baseline. After randomisation, the patients will be followed for one year within the frame of ReBioLup Part I, which is observational. Inclusion of patients participating in other investigator-initiated or pharmaceutical industry-driven therapeutic trials will be made possible, provided that those trials do not last more than 12 months. At month 12, ReBioLup patients will enter into Part II, which is interventional. A schematic flowchart of ReBioLup is presented on page 4.
In patients with 2003 ISN/RPS class III/IV (± V) at baseline and an NIH activity index score > 3 (cut-off based on a recent proof-of-concept retrospective analysis [Parodis I et al. Rheumatology (Oxford) 2020]) in the repeat kidney biopsy, the immunosuppressive therapy will be intensified based on the physician’s and patient’s shared decision (see recommendations below). In cases of pure membranous nephritis (ISN/RPS class V) at baseline which transformed to ISN/RPS class III/IV (± V) in the repeat biopsy, the same algorithm will be applied.
In patients with pure membranous (2003 ISN/RPS class V) LN in the repeat biopsy, individual assessment of the biopsy should steer the decision of treatment.
Patients who have not undergone a repeat biopsy will be treated according to standard clinical parameters. Percentages of complete renal response at month 24 and renal impairment at month 60 will be compared between the two study arms.